
宠立维®(头孢氨苄) 产品介绍
主要成分: 头孢氨苄
性状: 本品为类白色至微黄色片。
适应证: 用于治疗犬的革兰氏阳性菌和阴性菌感染,如皮肤感染(脓皮病、毛囊炎、蜂窝组织炎等)。
用法与用量: 以头孢氨苄计。内服:一次量,每1kg体重 犬15mg。一日2次,治疗尿路感染连用14天;浅表脓皮病连用7~14天;深层脓皮病连用28天。
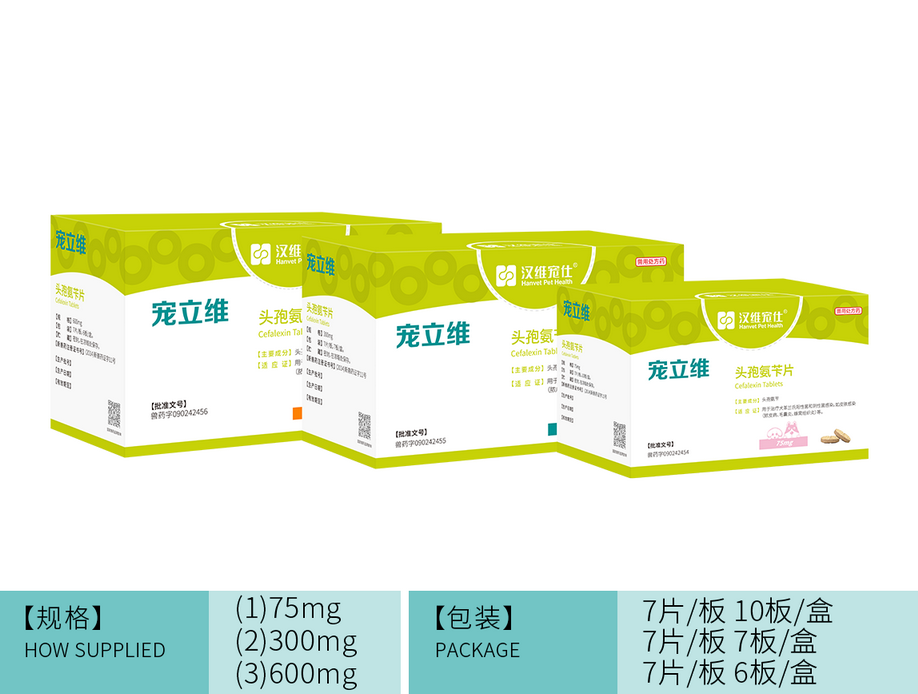
规格: 75mg 300mg 600mg
包装: 75mg 7片/板,10板/盒
300mg 7片/板,7板/盒
600mg7片/板,6板/盒
贮藏: 遮光,密封保存。
新兽药注册证书号: (2014)新兽药证字11号
批准文号: 兽药字090242454 090242455 090242456
Trolevis Product Info
ACTIVE INGREDIENT:
Cefalexin (as cefalexin monohydrate)
PHARMACEUTICAL FORM:
Tablet ,White to light yellow, palatable tablet.
INDICATIONS FOR USE:
Dog: For the treatment of bacterial skin infections in dogs (including deep and superficial pyoderma) caused by organisms sensitive to cefalexin.For the treatment of urinary tract infections in dogs (including nephritis and cystitis) caused by organisms sensitive to cefalexin.Cat: Infections caused by bacteria susceptible to cefalexin. Lower urinary tract infections due to E.coli and Proteus mirabilis. Treatment of cutaneous and subcutaneous infections: pyoderma due to Staphylococcus.spp and wounds and abscesses due to Pasteurella spp.
HOW SUPPLIED PACKAGE:
(1) 75mg (2) 300mg (3) 600mg
PACKAGE:
(1) 75mg Cardboard box with 10 blister of 7 tablets
(2) 300mg Cardboard box with7 blister of 7 tablets
(3) 600mg Cardboard box with6 blister of 7 tablets
STORAGE INFORMATION:
Store in the original package. Keep out of light.
REGISTERED NUMBER OF APPROVAL:
(2014) New Veterinary Drug Certificate No.11
LICENSE NUMBER:
Veterinary Drug License 090242454 090242455 090242456

宠立维®(头孢氨苄) 产品特点
• 宠物专用,剂量精准,适口性好;
• 犬口服后1小时即可达血液峰值浓度;
• 抗菌谱广,可用于大多数革兰氏阳性菌和革兰氏阴性菌;
• 渗透性强,可歼灭组织深部的顽固细菌;
• 生物利用度高,试验证明口服生物利用度高达90%;
• 动物耐受度高,可长期用药;
Trolevis Product Features
• Dedicated for pet use with accurate dose and good palatability.
• Rapid uptake. The highest peak blood concentration will be reached within one hour after oral administration and therapeutic effect to canine skin will be achieved within 2 hours.
• With broad antibacterial spectrum, it is high effective to the majority of Gram-positive and Gram-negative bacterium.
• Having good tissue penetration, it can exterminate pertinacious bacteria in the deep tissue.
• Showing high bioavailability, it proved that oral bioavailability can reach 97% in experiments .
• Because of mild side effects and good tolerance, it can be for long term use.
宠立维®(头孢氨苄) 常见问题
1.宠立维适应症?
用于治疗犬的革兰氏阳性菌和阴性菌感染,如皮肤感染(脓皮病、毛囊炎、蜂窝组织炎等)
Trolevis Q&A
1.INDICATIONS FOR USE?
Dog: For the treatment of bacterial skin infections in dogs (including deep and superficial pyoderma) caused by organisms sensitive to cefalexin.For the treatment of urinary tract infections in dogs (including nephritis and cystitis) caused by organisms sensitive to cefalexin.Cat: Infections caused by bacteria susceptible to cefalexin. Lower urinary tract infections due to E.coli and Proteus mirabilis. Treatment of cutaneous and subcutaneous infections: pyoderma due to Staphylococcus.spp and wounds and abscesses due to Pasteurella spp.
宠立维®(头孢氨苄) 注意事项
1.禁用于对青霉素类药物过敏的动物;
2.禁用于兔子、豚鼠、沙鼠和仓鼠;
3.肾功能受损的动物同时服用其他经肾排泄的药物会加重本药在体内的蓄积,因此在动物肾功能不全时可减少本药的用量;
4.对青霉素类过敏的人不得接触该产品;
5.使用该品时要倍加小心以避免直接接触,使用后要洗手;
6.给药人员皮肤出现红疹时要尽快就医,若嘴唇、眼睑和脸部肿胀、呼吸困难要立即拨打急救电话。
Trolevis Attention
1.Do not use in animals which are known to be hypersensitive to penicillins and cephalosporins.
2.Do not use in rabbits, guinea pigs, hamsters and gerbils.
3.As with other antibiotics which are excreted mainly by the kidneys, unnecessary accumulation may occur in the body when renal function is impaired. In case of known renal insufficiency the dose should be reduced.
4.Do not handle this product if you know you are sensitised, or if you have been advised not to work with such preparations.
5.Handle this product with great care to avoid exposure, taking all recommended precautions. Wash hands after use.
6.If you develop symptoms following exposure such as skin rash you should seek medical advice and show the doctor this warning. Swelling of the face, lips or eyes or difficulty breathing are more serious symptoms and require urgent medical attention.
 |