
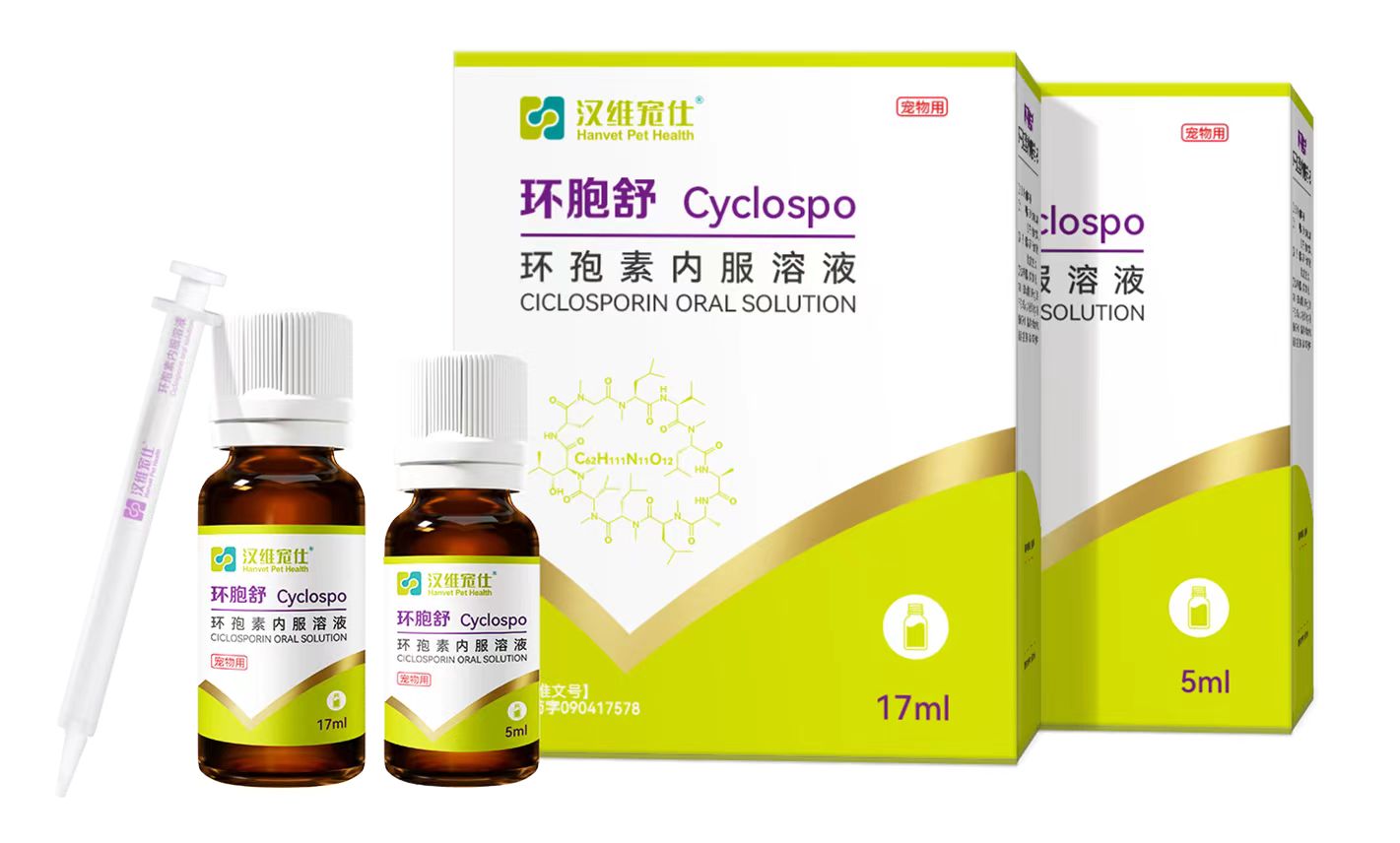
环胞舒® 产品介绍
主要成分(ACTIVE INGREDIENT): 环孢素(Cyclosporin)
性状(PHARMACEUTICAL FORM):
本品为淡黄色或黄色的澄清油状液体。
(This product is a light yellow or yellow clarified oily liquid.)
适应症(INDICATIONS FOR USE):
本品用于治疗猫慢性过敏性皮炎。
This product is used to treat chronic allergic dermatitis in cats.
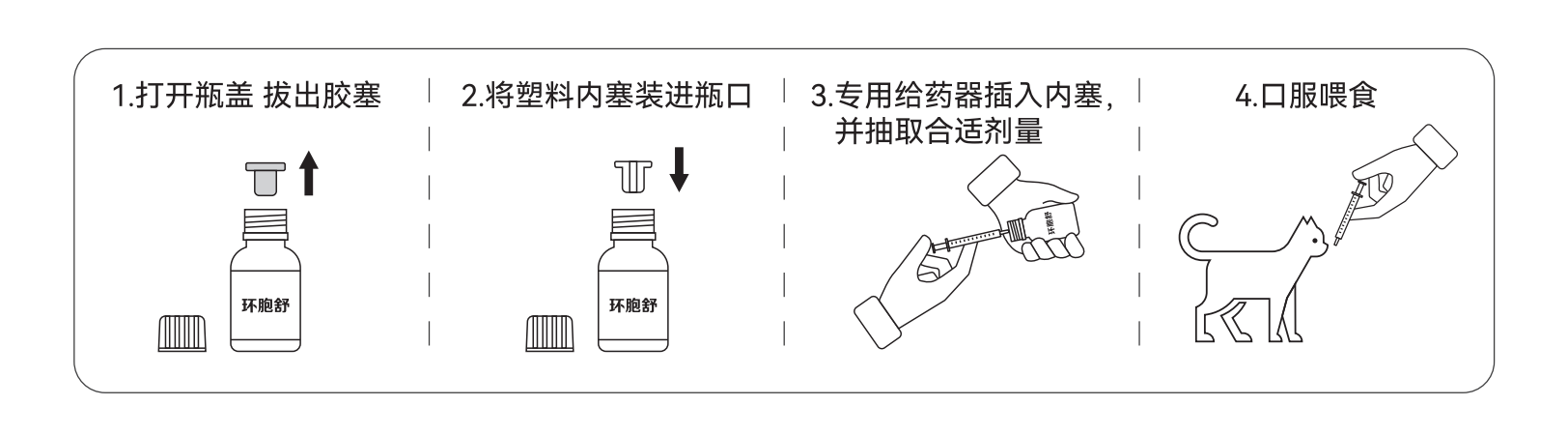
用法用量 DOSAGE AND ADMINISTRATION:以有效成分计 。 内服: 每 1kg 体重 ,猫 7mg
(相当于以本品计 。 内服 :每 1kg体重 ,猫 0.07 ml)。
In terms of active ingredient. Internal use: 7mg of cat per 1kg of body weight.
(Equivalent to the product. Internal: 0.07 ml per 1 kg body weight in cats.)
规格(HOW SUPPLIED PACKAGE):
5mL/瓶、17mL/瓶 ( 5mL/17mL per bottle. )
包装(PACKAGE):
1瓶 / 盒。( 1 bottle per box . )
贮存(STORAGE INFORMATION):
15~30℃密封保存 ,避免冷藏 。不要在低于20℃的条件下贮藏超过一个月。
( Keep sealed at 15-30℃ and avoid refrigeration. Do not store at less than 20℃ for more than one month.)
环胞舒® 产品特点
抗炎调免疫,控制原发病;
• 10+免疫病,适用范围广;
• 自微乳释药,纳米级好吸收;
• 宠物友好口服液,剂量精准更安全。
Cyclospo® Product Features
- Anti-inflammatory modulation of immunity and control of primary disease;
- 10+ immune diseases can be used, with a wide range of application;
- Self-microemulsion drug release, nanoscale good absorption;
- Pet-friendly oral solution, accurate and safer dosage.
常见问题
1.药物的使用周期(以过敏性皮炎为例)
治疗初期,本品应每日给药,直至观察到满意的临床症状改善(通常 4~8 周内)。应定期重新评估患猫并复核治疗方案。
一旦过敏性皮炎的临床体征得到满意控制,本品可每隔一天给药一次。在每隔一天给药一次,即可控制临床症状的一些病例中,兽医可每 3~4 天给予本品一次。当临床症状得到控制时可停止治疗。当临床症状复发时,应恢复至每日给药进行治疗,在某些情况下,可能需要进行重复治疗。
1. Periodicity of use of the drug (in the case of atopic dermatitis)
In the initial stage of treatment, this product should be administered daily until satisfactory clinical improvement is observed (usually within 4 to 8 weeks). The affected cat should be reassessed and the treatment regimen reviewed periodically.
Once the clinical signs of atopic dermatitis have been satisfactorily controlled, the product may be administered every other day. In some cases where clinical signs can be controlled by every other day administration, the veterinarian may give the product every 3 to 4 days. Treatment may be discontinued when clinical signs are under control. When clinical signs recur, treatment should be resumed on a daily basis and, in some cases, repeat treatment may be necessary.
2. 环孢素安全使用年龄及体重范围。
农业部标签说明书:尚未在 <6 月龄或体重< 2.3kg 的猫中评估环孢素的有效性和安全性。不得用于 6 月龄以下或体重低于 2.3kg 的猫。
国际最新研究:环孢素适用于6 月龄以上且体重大于1.4kg的猫。适用于体重大于1.8kg的犬。
2. Age and weight range for safe use of cyclosporine.
USDA Labeling Statement: The efficacy and safety of cyclosporine have not been evaluated in cats <6 months of age or weighing < 2.3 kg. It should not be used in cats <6 months of age or weighing < 2.3 kg.
Latest international studies: Cyclosporine is indicated for cats over 6 months of age and weighing >1.4kg. For dogs weighing more than 1.8kg.
3. 可能的不良反应?
胃肠道症状,如呕吐和腹泻。这些症状通常是轻微和短暂的,不需要停止治疗。
· 本品治疗期间可能发生食欲下降和体重减轻,建议监测体重。体重显著降低可
能导致肝脏脂质沉积。
· 如果在治疗期间发生持续的、渐进性体重减轻,建议停止治疗,直至确定原因。
3. Possible adverse reactions?
Gastrointestinal symptoms such as vomiting and diarrhea. These symptoms are usually mild and transient and do not require discontinuation of treatment.
- Decreased appetite and weight loss may occur during treatment with this product and weight monitoring is recommended. Significant weight loss may
A significant decrease in body weight may result in lipid deposition in the liver.
- If persistent, progressive weight loss occurs during treatment, it is recommended that treatment be discontinued until the cause is determined.
环胞舒® 注意事项
1. 本品不得用于感染白血病病毒( FeLV )或免疫缺陷病毒( FIV )的猫。
2. 本品不得用于有恶性疾病史或进行性恶性疾病史的猫。
3. 尚未在 6 月龄以下或体重低于 2.3kg 的猫中评估环孢素的有效性和安全性 。不得用于 6 月 龄以下或体重低于 2.3kg 的猫。
4. 尚未在雄性种猫或在妊娠期或哺乳期母猫中研究药物的安全性 。在用于繁殖期、妊娠期或 哺乳期的猫之前 ,应进行全面的风险/效益分析。
5. 环孢素可能引起血糖水平升高 。尚未评价环孢素对患有糖尿病的猫的影响 。患有糖尿病的 猫不建议使用环孢素。
6. 环孢素虽然不诱发肿瘤,但可以抑制 T 淋巴细胞 ,因此用环孢素治疗可能增加临床上明显 的恶性肿瘤的发生率 。如果在接受环孢素治疗的猫中观察到淋巴结病变 ,建议进行进一步的临床 研究 ,必要时停止治疗。
7. 多种药物可竞争性抑制或诱导参与环孢素代谢的酶 ,尤其是细胞色素 P450(CYP 3A 4)。 唑类化合物(如酮康唑)可增加猫体内环孢素的血药浓度 。大环内酯类药物(如红霉素)可使血 浆环孢素水平增加两倍 。细胞色素 P450 的某些诱导剂、抗惊厥药和抗生素(如甲氧苄啶、磺胺嘧 啶)可降低环孢素的血药浓度。
8. 环孢素作为 MDR l P–糖蛋白转运载体的底物和抑制剂 ,与 P–糖蛋白底物(如大环内酯 类)联合给药可降低这些药物从血脑屏障细胞内流出,可能导致中枢神经系统(CNS)毒性体征。 在临床研究中对猫采用环孢素和赛拉菌素或米尔贝肟联合治疗 ,没有发现这些药物联合运用与神 经毒性之间的相关性。
9. 环孢素可增加氨基糖苷类抗生素和甲氧苄啶的肾毒性。不建议环孢素与这些活性成分同时 使用。
10. 本品治疗可能引起疫苗接种的免疫应答降低 ,不建议在治疗期间或给药前后两周内接种 疫苗。
11. 对于弓形虫血清呈阴性的猫 ,在治疗期间感染时会有患上弓形虫病的风险 。在极少的情 况下可能会致命 。因此应尽量减少血清阴性猫接触弓形虫的可能性(例如 ,室内饲养 、避免生肉或腐食)。在一项对照实验研究中,发现环孢素没有引起弓形虫卵母细胞脱落的增加。在治疗中发 现弓形虫病或其他严重的全身性疾病时 ,应停止环孢素的使用并开始适当的治疗。
12. 由于环孢素具有免疫抑制作用 , 因此在开始治疗前应对任何其他的感染进行适当治疗。 治疗期间发生的感染无需停药 ,除非感染严重。
13. 不建议与其他免疫抑制剂同时使用。
14. 意外摄入本品可能导致恶心和/或呕吐 ,应立即就医 。应避免与眼睛接触 ,如不慎接触, 请用大量水冲洗。
15. 环孢素可能引起超敏(过敏)反应 。对环孢素过敏的人应避免接触本品。
16. 置儿童不可触及处。
Cyclospo® Attention
1. This product should not be used in cats infected with leukemia virus (FeLV) or immunodeficiency virus (FIV).
2. This product should not be used in cats with a history of malignant disease or progressive malignant disease.
3. The efficacy and safety of cyclosporine have not been evaluated in cats less than 6 months of age or weighing less than 2.3 kg. Cyclosporine should not be used in cats under 6 months of age or weighing less than 2.3 kg.
4. The safety of the drug has not been studied in male breeding cats or in pregnant or lactating female cats. Before using in breeding, pregnant or lactating cats, a comprehensive risk/benefit analysis should be performed.
5. Cyclosporine may cause elevated blood glucose levels. The effects of cyclosporine in diabetic cats have not been evaluated. Cyclosporine is not recommended for cats with diabetes mellitus.
6. Cyclosporine does not induce tumors, but it does inhibit T lymphocytes; therefore, treatment with cyclosporine may increase the incidence of clinically significant malignancies. If lymphadenopathy is observed in cyclosporine-treated cats, further clinical studies are recommended and treatment should be discontinued if necessary.
7. A variety of drugs can competitively inhibit or induce enzymes involved in the metabolism of cyclosporine, particularly cytochrome P450 (CYP 3A 4). Azoles (e.g., ketoconazole) can increase blood levels of cyclosporine in cats. Macrolides (e.g., erythromycin) can triple plasma cyclosporine levels. Certain inducers of cytochrome P450, anticonvulsants and antibiotics (e.g., methotrexate, sulfadiazine) can decrease cyclosporine blood levels.
8. Cyclosporine acts as a substrate and inhibitor of the MDR l P-glycoprotein transporter, and co-administration of cyclosporine with P-glycoprotein substrates (e.g., macrolides) reduces the intracellular efflux of these drugs from the blood-brain barrier, which may result in signs of central nervous system (CNS) toxicity. No correlation between the combination of cyclosporine and selamectin or milbemycin in cats and neurotoxicity was found in clinical studies.
9. Cyclosporine may increase the nephrotoxicity of aminoglycoside antibiotics and methotrexate. Concomitant use of cyclosporine with these active ingredients is not recommended.
10. Treatment with this product may cause a reduction in the immune response to vaccination and vaccination is not recommended during treatment or within 2 weeks before or after administration.
11. Toxoplasma gondii seronegative cats are at risk of developing toxoplasmosis if infected during treatment. In rare cases this may be fatal. Exposure of seronegative cats to Toxoplasma gondii should therefore be minimized (e.g., indoor housing, avoidance of raw meat or carrion). In a controlled experimental study, cyclosporine was not found to cause an increase in Toxoplasma gondii oocyte shedding. If toxoplasmosis or other serious systemic disease is detected during treatment, cyclosporine should be discontinued and appropriate therapy initiated.
12. Because of the immunosuppressive effect of cyclosporine, any other infections should be appropriately treated prior to initiating therapy. Infections occurring during treatment should not be discontinued unless they are severe.
13. Concomitant use with other immunosuppressive agents is not recommended.
14. Accidental ingestion of this product may result in nausea and/or vomiting; seek medical attention immediately. Avoid contact with the eyes; if contact occurs, flush with plenty of water.
15. Cyclosporine may cause hypersensitivity (allergic) reactions. Avoid contact with this product if you are allergic to cyclosporine.
16. Keep out of reach of children.
 |